120409salt.jpg
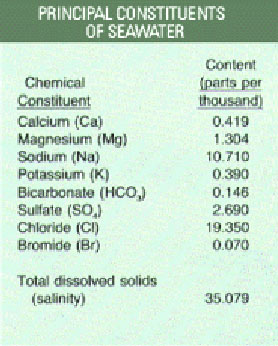
Principle constituents of seawater. Credit: wikipedia
Water, water everywhere -- and not a drop to drink. That’s a key plot point in any tale of survival at sea. Even though the heroes are floating atop an ocean, they can’t drink the water because it’s too salty.
Seawater is about 200 times saltier than freshwater. You’d have to stir a teaspoonful of table salt into a glass of tap water to equal the salt content of the oceans.
Ocean “salt” contains a variety of chemical elements. Sodium and chloride -- the ingredients of common table salt -- account for almost 90 percent of these dissolved elements.
Many of these elements are carried out to sea by rivers and streams. Rain, winds, and other processes chip away the rocks on land, and many of the eroded particles find their way into waterways. The rivers and streams deposit several billion tons of new salts into the oceans every year.
Another source of these elements is the mid-ocean ridges -- sections of the ocean floor that are spreading apart to form new crust. Ocean water reacts with hot rocks in the ridges, and carries away lots of particles.
Even though new particles are constantly added to the oceans, the level of salinity most likely has remained steady for billions of years. That’s because some of the heavier particles settle on the ocean floor, eventually forming rock beds, while living organisms use calcium and other elements to make their hard shells. So while the dissolved particles in seawater are harmful to people, they’re a critical resource for life in the oceans.

