On summer days here in Texas, the TV weather folks often tell us that the mercury once again topped a hundred degrees -- a reference to the days when many thermometers were glass tubes filled with liquid mercury.
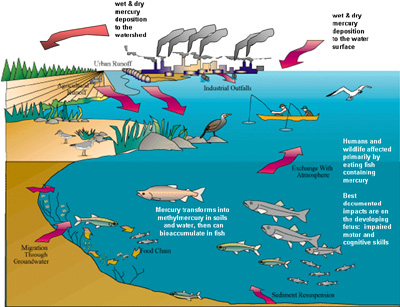 A depiction of mercury cycling. Credit: National Oceanic and Atmospheric Administration.
A depiction of mercury cycling. Credit: National Oceanic and Atmospheric Administration.The metal has been phased out of thermometers and many other products, though, because it’s toxic. But it’s still all around us -- in the soil, the air, the water, and even in many of the big marine fish we eat.
Scientists aren’t yet sure just how the mercury gets into the fish. But they suspect that bacteria in the water play a key role.
Mercury is a natural element that’s found all across the planet. Some of it enters the air and water through volcanoes or through erosion of rocks that contain high concentrations of the element. But today, a lot of mercury comes from industry, such as some types of coal-fired power plants, as well as gold mines, which use mercury in refining the ore.
Some of the mercury washes into the oceans, or the oceans absorb it from the air. It settles to fairly deep layers, where bacteria eat dead organic matter that’s fallen from the surface. As part of this process, the bacteria may convert mercury in the water into a highly toxic form, known as methylmercury. Larger organisms eat the bacteria, which in turn get eaten by even larger organisms all the way up the food chain. This process can concentrate the amount of methylmercury found in large fish, such as tunas and swordfish -- a potential danger to the people who eat them. More about that on our next program.

